Physician's Notebooks 2 - http://physiciansnotebook.blogspot.com - See Homepage
Preface to Food Nutrients: The following sections have in mind a presentation of the food nutrients - the carbohydrates, lipids, proteins, and vitamins & minerals - first off as chemicals. I want it to lead into the functions of food chemicals in good health for the average reader wishing for healthy longevity. Each reader may also have his or her own focus they may wish to concentrate on. OK, we start with the carbohydrates.
2.9a: The Caloric Nutrients - Carbohydrates (Update 20 Nov. 2021). The following are the main headings in order in text.
Preface to Food Nutrients: The following sections have in mind a presentation of the food nutrients - the carbohydrates, lipids, proteins, and vitamins & minerals - first off as chemicals. I want it to lead into the functions of food chemicals in good health for the average reader wishing for healthy longevity. Each reader may also have his or her own focus they may wish to concentrate on. OK, we start with the carbohydrates.
2.9a: The Caloric Nutrients - Carbohydrates (Update 20 Nov. 2021). The following are the main headings in order in text.
Molecular structure
Carbohydrate is our manna from heaven
Types of Carbohydrate
Hyperglycemia & Hypoglycemia
The Common Food Sugars
The most common food carbohydrate, starch
Digestion & Absorption
Surviving in a Famine
Insulin's Role
Storage in Body
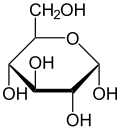
"Carbohydrate", because molecular structure shows carbohydrates to be made of C (Carbon hence “carbo”), and H2O (water, hence “-hydrate”). The basic units of carbohydrates are the sugars. Glucose (Note the -ose ending, denoting a sugar, e.g., lactose, the sugar in milk) is the primary natural sugar. All carbohydrates get digested or metabolized down to the basic glucose structure and then it is used mostly to produce energy but also can be used as part of structures. The simplest chemical expression of Glucose sugar is C6H12O6. It shows the ratio of carbon C, hydrogen H and oxygen O atoms in a single molecule of Glucose. But Glucose actually exists as the 3-D, cyclic structure molecule
Note the molecule's 6-sided hexagon shape with one atom Oxygen, O, at one of the apexes of the hexagon and atoms of Carbon, C, at all the other apexes (In the above structure, the C's at the other apexes of the hexagon are not shown but should be visualized by the reader). Also there is a numbering system, not shown above that will help classify the different 1-sugar carbohydrates. Starting from the apex O oxygen atom, you count the carbon atoms in a clockwise direction around the hexagon beginning immediately to the reader's right with C1 and counting C2, C3, C4, C5 for all the apex C's and then C6 in the CH2OH C atom that juts out of the top of the hexagon to your right.
The glucose structure is important for digestion and metabolism because the 3-D structure is acted upon by enzymes like key in lock.Carbohydrate is our manna from heaven; its ultimate source comes from photosynthesis in green plants: the carbon dioxide (CO2) in Earth's atmosphere is the source of the C carbon that is combined with water (H2O) by the energy in our Sun's rays (Photons), the reaction being sped by the enzyme chlorophyll. The full reaction is
CO2 + H2O + Photons (Chlorophyll)---->Cn(H2O)n (Carbohydrate) + O2.
At one swoop, the chlorophyll in green leaf converts the sun's ray's into energy to produce a basic food substance (carbohydrate), clears the atmosphere of the greenhouse gas CO2 and supplies our breathing oxygen O2.
Types of Carbohydrate: The general structure for carbohydrate is Cn(H2O)n. Simplest is the sugars that are monosaccharides ("mono-," one or single, and “-saccharide,” sugar), most kinds of which are molecules where the above subscript n is either 6 or 5. In the 6-C sugars “hexoses”, the “hex-“ is prefix for the number 6. These are important in producing the energy from food that allows us to move and function. The sugar Glucose, whose properties are determined by its 3-D structure (See above), is the most important natural sugar.Hyperglycemia & Hypoglycemia: Glucose in blood when high is hyperglycemia, as seen in diabetes mellitus, and when too low due to a diabetic injecting self or other with excess insulin is hypoglycemia, which causes cold-sweat, fainting and, if prolonged, death. For best body functioning and no unpleasant symptoms, one needs artery blood glucose concentrations between 60 and 120 mg% (3.3 to 6.6 mM/L).The Common Food Sugars Our food also has the 6-C monosaccharide sugar, fructose (as name suggests, it is sugar of fruit), and galactose (in milk). The 3 monosaccharides – glucose, fructose, galactose – are molecules that in simple chemical notation are C6H12O6, and each simple monosaccharide differs from other only in 3-D and cyclic structure.Food sugars of 2 each monosaccharides are disaccharides (“di” = Greek “two”). Sucrose table sugar is the disaccharide made of a molecule each of glucose and fructose. Maltose sugar of plant stems is disaccharide of 2 linked glucoses, and Lactose sugar of milk is disaccharide of glucose and galactose.The most common food carbohydrate, starch, is composed of very large molecules, each molecule of starch consisting of hundreds of monosaccharides. This carbohydrate is called “polysaccharide” ("poly-" is the prefix for many, in English, multi-). Starches differ from each other in number of monosaccharides, in the branching in the molecule, and in the kind of chemical bonding. The purpose of starches is storage of the energy in food. In humans, the equivalent of starch is glycogen, which is formed and stored in liver, muscle and brain when an excess of glucose gets produced from the diet. Glycogen allows us to go without food for days as a source of internal energy by getting reconverted into glucose and burned as fuel (metaphoric for glucose getting oxidized and releasing its ATP storage packets).Understanding carbohydrate at level of molecule – from polysaccharide as starch or glycogen, disaccharide as common form of food sugars, and monosaccharide 6-C sugar unit – is key to the digestion and utilization of carbohydrate. We eat the polysaccharides and they get digested quickly in mouth and stomach to the disaccharide sugars, and then to fructose, galactose (from milk) and glucose, and these 3 monosaccharides are absorbed into blood and transported to liver, where the galactose and fructose are converted to glucose the final carbohydrate fuel unit. Of course we may also eat di- and mono-saccharides directly with the same final effect to convert to glucose in liver.
Here is where “manna from heaven” comes in. Carbohydrate is the original food substance. Plants form it from air and water, using sun’s energy plus chlorophyll in green leaf. An animal cannot make it from simpler molecules. Most calories we need to move and much of our structural substance is produced, fueled by, and comes from the body’s conversion of glucose into energy and into other chemical compounds. So an animal must eat plants or must eat other animals that eat plants to continue to live. From glucose, many other nutrients are made by living things. Many living forms live purely on the carbohydrates, which are closest to a universal food.
There are also 5-carbon sugars called pentoses whose structure is essentially the same as the 6-carbon variety but whose basic units are 5-carbon saccharides. They may be part of enzymes or other structural units. Some genetic disease involve them.
To read following is Section B on Lipids now, clickDigestion & Absorption of starch as for example bread or rice, can start with cutting into small pieces and cooking. Good boiling (15 min with 30-min steep), especially in slightly acid solution (a little vinegar to pot) will start conversion of starch to sugar. The digestion of starch actually starts or continues in mouth as you chew because saliva contains enzyme that breaks down starch molecule to sugar monosaccharides. Chewing slowly and well is good for the digestion.In stomach and upper intestine, the carbohydrates - starches and disaccharide food sugars – digest to the 6-C saccharides which can pass thru pores in wall in upper small intestine into venous blood that goes to liver.Surviving in a Famine If you know carbohydrate digestion, it will come in handy in a famine because the most important carbohydrate food is not normally available since we humans – unlike cow and other ruminant – lack ability to digest starch-like cellulose, the structural substance of plants. But, if you recall that a complex carbohydrate can be digested by boiling in slightly acid solution, and apply that by boiling (with a little vinegar if you have it) bark, leaf, grass, and wood that is cut up into very small pieces next time you are caught in a famine, you will be rewarded by getting sweet soup and it will earn you a “Corky” for popping up long after others sink.Insulin's Role With the help of insulin produced by special cells in the pancreas, the glucose enters cells and either breaks down and combines with oxygen in a metabolic cycle to produce phosphate bond packets of energy (ADP to ATP and the ATP releases high energy packet per ATP to ADP broken down phosphate bond) to energize the body or else is turned into or combined with structural units of starch, fat or protein.
2.9b Fats or Lipids in Foods
No comments:
Post a Comment